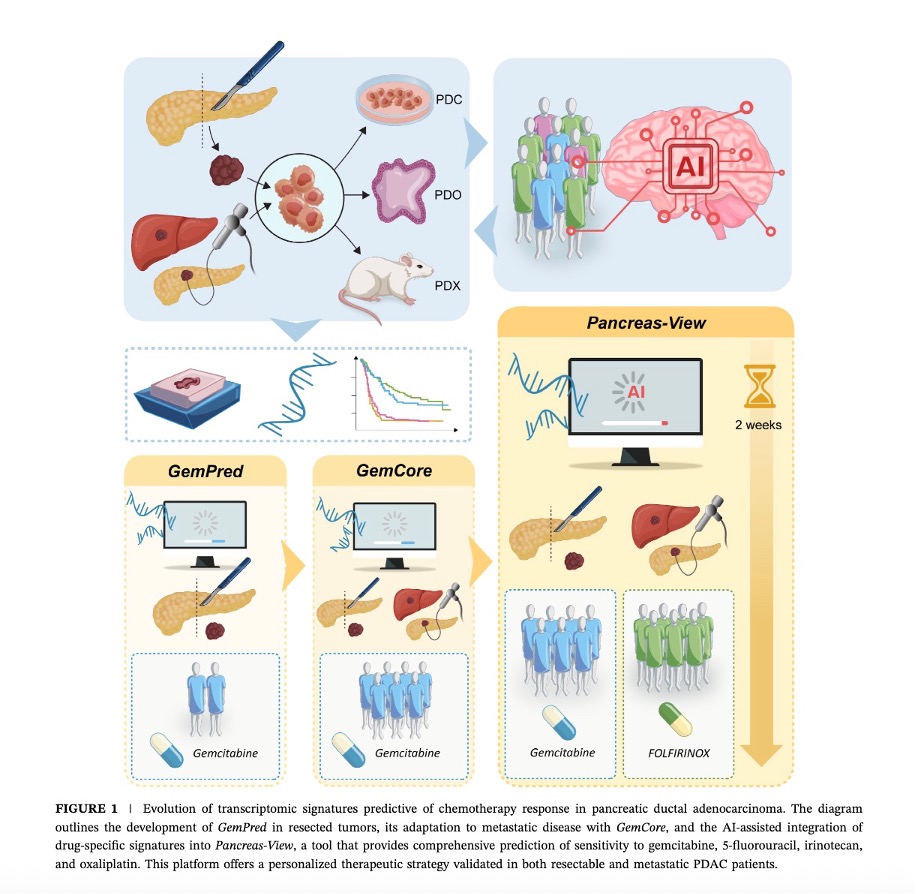
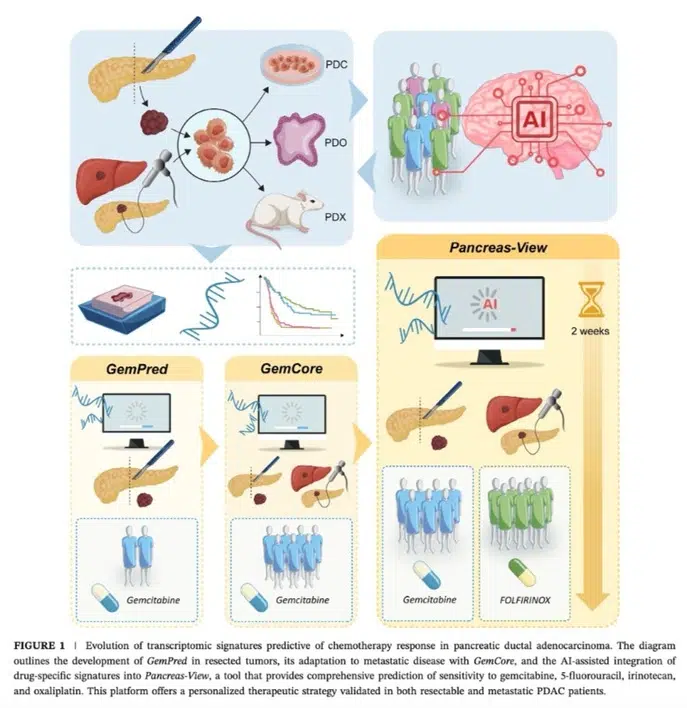
Our team focuses on the molecular aspects of pancreatic cancer initiation and progression in order to improve currently available therapeutic approaches.
Pancreatic cancer is one of the leading causes of cancer deaths worldwide. In 2020, some 466,000 people died from it. Despite advances in research, the prognosis remains bleak, with a five-year net survival of around 11% for cases diagnosed between 2010 and 2015. This high case-fatality rate is explained by the often late diagnosis, limited treatment options, aggressive nature and notable resistance to chemotherapy. In addition, significant heterogeneity is observed among patients, resulting from varied genetic mutations, complex interactions with the tumor microenvironment and selection pressure leading to clonal expansion.
Our team focuses on the molecular aspects of pancreatic cancer development and progression in order to improve current therapeutic approaches. Our research focuses on three main areas:
- Signaling and metabolism: studying NUPR1 targeting for the treatment of PDAC (Juan Iovanna and Patricia Santofimia-Castaño).
- Dialogues between adhesion molecules in pancreatic cancer: studying the expression profile of cadherins and their role in tumor invasion (Frédéric André and Véronique Rigot).
- Translational research: investigating the development of personalized treatments for PDAC (Nelson Dusetti and Juan Iovanna), the role of abnormal glycosylation processes in PDAC progression (Eric Mas), the role of post-translational modifications (PTMs) in PDAC biology and resistance mechanisms (Philippe Soubeyran), and the targeting of resistant PDAC phenotypes (Nicolas Frauhoffer).
The projects
Project members :
NUPR1, an intrinsically disordered nuclear protein (IDP), plays a crucial role in pancreatic cancer (PDAC) and other types of cancer. NUPR1 plays a crucial role in regulating tumor growth, and its genetic inactivation results in a complete halt to tumor development. Due to the unique structural characteristics of intrinsically disordered proteins, the development of specific inhibitors for NUPR1 remains a major challenge. Our team is tackling this problem using innovative, multidisciplinary approaches. Our research has led to the discovery of ZZW-115, a potent anticancer compound tested in vitro and in vivo. Using ZZW-115, we observed dose-dependent tumor regression in xenograft mice, with no apparent side effects. Our cellular analyses revealed that ZZW-115 induced necroptosis, apoptosis and ferroptosis in response to mitochondrial destabilization, highlighting its therapeutic potential. Our most recent results show that ZZW-115 inhibits the formation of NUPR1-dependent stress granules, thereby slowing the progression of pancreatic intraepithelial neoplasia (PanINs) in mice carrying the oncogenic KrasG12D mutation. This inhibition led to cell death, caspase 3 activation, DNA fragmentation and the formation of apoptotic bodies after four weeks' treatment. Our team continues to explore these mechanisms to offer new therapeutic perspectives in the fight against NUPR1-dependent cancers.
Ongoing research is focusing on ZZW-115's mechanism of action, NUPR1's molecular partners, its synergy with DNA-damaging agents and regulatory preclinical studies. The ZZW-115 compound has been granted patent protection, and a start-up company (PanCa Therapeutics) has been set up to take it into clinical trials.
- Targeting NUPR1-dependent stress granule formation to induce synthetic lethality in KrasG12D-driven tumors. Santofimia-Castaño P, Fraunhoffer N, Liu X, Bessone IF, di Magliano MP, Audebert S, Camoin L, Estaras M, Brenière M, Modesti M, Lomberk G, Urrutia R, Soubeyran P, Neira JL, Iovanna J. EMBO Mol Med. 2024 Feb 15.
- NUPR1 protects against hyperPARylation-dependent cell death. Santofimia-Castaño P, Huang C, Liu X, Xia Y, Audebert S, Camoin L, Peng L, Lomberk G, Urrutia R, Soubeyran P, Neira JL, Iovanna J. Commun Biol. 2022 Jul 22;5(1):732.
- NUPR1 inhibitor ZZW-115 induces ferroptosis in a mitochondria-dependent manner. Huang C, Santofimia-Castaño P, Liu X, Xia Y, Peng L, Gotorbe C, Neira JL, Tang D, Pouyssegur J, Iovanna J. Cell Death Discov. 2021 Oct 1;7(1):269.
- ZZW-115-dependent inhibition of NUPR1 nuclear translocation sensitizes cancer cells to genotoxic agents. Lan W, Santofimia-Castaño P, Swayden M, Xia Y, Zhou Z, Audebert S, Camoin L, Huang C, Peng L, Jiménez-Alesanco A, Velázquez-Campoy A, Abián O, Lomberk G, Urrutia R, Rizzuti B, Geli V, Soubeyran P, Neira JL, Iovanna J. JCI Insight. 2020 Sep 17;5(18):e138117.
- Ligand-based design identifies a potent NUPR1 inhibitor exerting anticancer activity via necroptosis. Santofimia-Castaño P, Xia Y, Lan W, Zhou Z, Huang C, Peng L, Soubeyran P, Velázquez-Campoy A, Abián O, Rizzuti B, Neira JL, Iovanna J. J Clin Invest. 2019 Mar 28;129(6):2500-2513.
Project members :
- 1. We determine a "Cadherin" signature corresponding to the expression profile of cadherins in relation to an invagram (3D invasiveness of tumor cells, ability to form invadopodia, etc.). The aim of this approach is to predict the aggressiveness of PDAC, enabling personalized patient follow-up.
- 2. We have identified a cadherin whose high expression is associated with a very poor prognosis in patients. We are trying to understand its functional involvement in tumor aggressiveness. Therapeutic and prognostic tools targeting this molecule are currently under development.
- 3. We are attempting to characterize whether the nature of the cadherins expressed by tumor cells has a qualitative and quantitative impact on the composition of the extracellular matrix.
- E-cadherin is a structuring component of invadopodia in pancreatic cancerDobric A, Germain S, Silvy F, Bonier R, Audebert S, Camoin L, Dusetti N, Soubeyran P, Iovanna J, Rigot V, André F. 2020/10/9, bioRxiv, 2020.10.09.332783.
- Cadherin-1 and cadherin-3 cooperation determines the aggressiveness of pancreatic ductal adenocarcinoma. Siret C, Dobric A, Martirosyan A, Terciolo C, Germain S, Bonier R, Dirami T, Dusetti N, Tomasini R, Rubis M, Garcia S, Iovanna J, Lombardo D, Rigot V, André F. Br J Cancer. 2018 Feb 20;118(4):546-557.
The expression profile and localization of cadherins are modulated during PDAC progression. We analyze the involvement of different cadherins in the invasion/metastasis cascade. Our objectives are to define a cadherin signature that accounts for the aggressiveness of PDAC and to develop a therapeutic target for at least one of them.
Project members :
We have validated the efficacy of these signatures in several retrospective studies, and our current work focuses on their clinical validation in prospective cohorts. The Pancreas-View algorithm, protected by a patent, has given rise to a start-up company, Predicting Med, created to ensure its industrial development. This initiative aims to integrate this innovative tool into routine clinical practice, in order to improve the management of pancreatic cancers.
- Development and validation of AI-assisted transcriptomic signatures to personalize adjuvant chemotherapy in patients with pancreatic ductal adenocarcinoma. Fraunhoffer N, Hammel P, Conroy T, Nicolle R, Bachet JB, Harlé A, Rebours V, Turpin A, Ben Abdelghani M, Mitry E, Biagi J, Chanez B, Bigonnet M, Lopez A, Evesque L, Lecomte T, Assenat E, Bouché O, Renouf DJ, Lambert A, Monard L, Mauduit M, Cros J, Iovanna J, Dusetti N. Ann Oncol. 2024 Sep;35(9):780-791. doi: 10.1016/j.annonc.2024.06.010. Epub 2024 Jun 19. PMID: 38906254
- Prediction of Adjuvant Gemcitabine Sensitivity in Resectable Pancreatic Adenocarcinoma Using the GemPred RNA Signature: An Ancillary Study of the PRODIGE-24/CCTG PA6 Clinical Trial. Nicolle R, Bachet JB, Harlé A, Iovanna J, Hammel P, Rebours V, Turpin A, Ben Abdelghani M, Wei A, Mitry E, Lopez A, Biagi J, François E, Artru P, Lambert A, Renouf DJ, Monard L, Mauduit M, Dusetti N, Conroy T, Cros J. J Clin Oncol. 2023 Nov 14:JCO2202668.
- A Transcriptomic-Based Tool to Predict Gemcitabine Sensitivity in Advanced Pancreatic Adenocarcinoma.Fraunhoffer N, Chanez B, Teyssedou C; Pdac Chemo Sensitivity Prediction Working Group; Iovanna JL, Mitry E, Dusetti NJ. Gastroenterology. 2023 Mar;164(3):476-480.e4.
- A transcriptomic signature to predict adjuvant gemcitabine sensitivity in pancreatic adenocarcinoma.Nicolle R, Gayet O, Duconseil P, Vanbrugghe C, Roques J, Bigonnet M, Blum Y, Elarouci N, Armenoult L, Ayadi M, de Reyniès A, Puleo F, Augustin J, Emile JF, Svrcek M, Arsenijevic T, Hammel P, Giovannini M, Grandval P, Dahan L, Moutardier V, Gilabert M, Van Laethem JL, Bachet JB, Cros J, Iovanna J, Dusetti NJ. Ann Oncol. 2021 Feb;32(2):250-260.
Project members :
The identification of resistant phenotypes in PDAC has revealed a multitude of molecules specifically modulated under these conditions. These molecules represent potential therapeutic targets as well as new biomarkers to better understand and counter treatment resistance. This project aims to explore these markers in detail using advanced preclinical models, such as patient-derived cell cultures (PDC), organoids (PDO) and human tumor-derived xenografts (PDX). By combining cutting-edge techniques such as CRISPR-Cas9 to specifically inhibit genes associated with chemoresistance, and epigenomic approaches to modulate their expression, we hope to transform resistant cells into susceptible ones. In addition, spatial and single-cell transcriptomic analyses identify the complex interactions between tumor epithelial cells and the tumor microenvironment (CAF, immune cells) in a treatment context. These analyses provide tools for predicting treatment responses and target specific cell subpopulations.By adopting this multidisciplinary approach, our aim is to develop innovative therapeutic strategies capable of overcoming tumor resistance, thereby improving the efficacy of chemotherapies and opening up new perspectives for the management of PDAC.
- Priming therapy by targeting enhancer-initiated pathways in patient-derived pancreatic cancer cells. Fraunhoffer NA, Moreno Vega AI, Abuelafia AM, Morvan M, Lebarbier E, Mary-Huard T, Zimmermann MT, Lomberk G, Urrutia R, Dusetti N, Blum Y, Nicolle R, Iovanna J. EBioMedicine. 2023 Jun;92:104602. doi: 10.1016/j.ebiom.2023.104602. Epub 2023 May 4. PMID: 37148583
- Inhibition of glucuronidation in pancreatic cancer improves gemcitabine anticancer activity. Fraunhoffer NA, Abuelafia AM, Chanez B, Bigonnet M, Gayet O, Roques J, Chuluyan E, Dusetti N, Iovanna J. Cancer Commun (Lond). 2022 Nov;42(11):1212-1216. doi: 10.1002/cac2.12365. Epub 2022 Sep 30. PMID: 36178036
- Multi-omics data integration and modeling unravels new mechanisms for pancreatic cancer and improves prognostic prediction.Fraunhoffer NA, Abuelafia AM, Bigonnet M, Gayet O, Roques J, Nicolle R, Lomberk G, Urrutia R, Dusetti N, Iovanna J. NPJ Precis Oncol. 2022 Aug 17;6(1):57. doi: 10.1038/s41698-022-00299-z. PMID: 35978026
Project members :
Ubiquitin-like post-translational modifications (ubiquitin and related proteins such as SUMOs and Nedd8) regulate the functions of all cellular proteins and are therefore involved in most of a cell's biological processes. Alterations in this type of TPM may be involved in oncogenic processes and be responsible for resistance mechanisms enabling the cancer cell to survive various anti-cancer treatments.Our goal is to identify these pancreatic cancer-associated alterations for use as markers of chemoresistance and as novel molecular targets for re-sensitization. Thus, restoring normal TPM of some of these targets has the potential to restore the sensitivity of pancreatic cancer cells to conventional therapies, thereby increasing their efficacy and hence patient survival.
- Pancreatic ductal adenocarcinoma ubiquitination profiling reveals specific prognostic and theranostic markers. El Kaoutari A, Fraunhoffer NA, Audebert S, Camoin L, Berthois Y, Gayet O, Roques J, Bigonnet M, Bongrain C, Ciccolini J, Iovanna JL, Dusetti NJ, Soubeyran P. EBioMedicine. 2023 Jun;92:104634.
- PML hyposumoylation is responsible for the resistance of pancreatic cancer. Swayden M, Alzeeb G, Masoud R, Berthois Y, Audebert S, Camoin L, Hannouche L, Vachon H, Gayet O, Bigonnet M, Roques J, Silvy F, Carrier A, Dusetti N, Iovanna JL, Soubeyran P. FASEB J. 2019 Nov;33(11):12447-12463.
Project members :
One of the main causes of PDAC resistance to treatment is the considerable genotypic and phenotypic variability between patient tumors, which involves a variety of complex molecular mechanisms. These include glycosylation processes, which are responsible for the appearance of abnormal glycosylated structures whose expression varies between patient tumors.These structures can be generated on glycoproteins and glycosphingolipids as a result of, among other things, profound alterations in the expression of glycosylation enzymes such as glycosyltransferases. These aberrant glycans thus make a major contribution to tumor heterogeneity and may participate in malignant transformation, tumor progression and metastatic dissemination.
We have recently shown that some of these glyco-genes can be correlated with the sensitivity or resistance of tumor cells to various therapeutic molecules.
Our project therefore aims to characterize the molecular and clinical impact of these glycosyltransferases and the aberrant glycans they produce. The aim is to determine their potential as histological glyco-biomarkers predictive of response to treatment. We are also exploring their role in the molecular mechanisms involved both in tumor progression and in modulating sensitivity or resistance to chemotherapies.
- A glycosyltransferase gene signature to detect pancreatic ductal adenocarcinoma patients with poor prognosis. Mohamed Abd-El-Halim Y, El Kaoutari A, Silvy F, Rubis M, Bigonnet M, Roques J, Cros J, Nicolle R, Iovanna J, Dusetti N, Mas E. EBioMedicine. 2021 Sep;71:103541.
Team news
featured publications
09/2024
Fraunhoffer N, Hammel P, Conroy T, Nicolle R, Bachet JB, Harlé A, Rebours V, Turpin A, Ben Abdelghani M, Mitry E, Biagi J, Chanez B, Bigonnet M, Lopez A, Evesque L, Lecomte T, Assenat E, Bouché O, Renouf DJ, Lambert A, Monard L, Mauduit M, Cros J, Iovanna J, Dusetti N.
03/2024
Santofimia-Castaño P, Fraunhoffer N, Liu X, Bessone IF, di Magliano MP, Audebert S, Camoin L, Estaras M, Brenière M, Modesti M, Lomberk G, Urrutia R, Soubeyran P, Neira JL, Iovanna J.
03/2023
Fraunhoffer N, Chanez B, Teyssedou C; Pdac Chemo Sensitivity Prediction Working Group; Iovanna JL, Mitry E, Dusetti NJ.
08/2022
Fraunhoffer NA, Abuelafia AM, Bigonnet M, Gayet O, Roques J, Nicolle R, Lomberk G, Urrutia R, Dusetti N, Iovanna J.
05/2021
Cano CE, José Sandí M, Hamidi T, Calvo EL, Turrini O, Bartholin L, Loncle C, Secq V, Garcia S, Lomberk G, Kroemer G, Urrutia R, Iovanna JL.
02/2021
Nicolle R, Gayet O, Duconseil P, Vanbrugghe C, Roques J, Bigonnet M, Blum Y, Elarouci N, Armenoult L, Ayadi M, de Reyniès A, Puleo F, Augustin J, Emile JF, Svrcek M, Arsenijevic T, Hammel P, Giovannini M, Grandval P, Dahan L, Moutardier V, Gilabert M, Van Laethem JL, Bachet JB, Cros J, Iovanna J, Dusetti NJ.


Labels, Funding and Partners
Like others, they were part of the team. Thank you to all those who have contributed to CRCM's excellence and impact.